Path-For-Young, an international project to personalise care for young women with breast cancer

 Led by Dr Ines Vaz Luis and coordinated by Unicancer, the Path-For-Young project aims to transform the management of young patients with breast cancer at high risk of relapse and to improve their quality of life after the disease. This innovative, large-scale programme is based on a major international multi-centre clinical trial and benefits from the collaboration of 26 partners, including cancer centres, universities, international cooperative research groups and patient associations based in 15 different countries. It is funded by the European Union to the tune of €9.5 million (grant agreement no. 101156800).
Led by Dr Ines Vaz Luis and coordinated by Unicancer, the Path-For-Young project aims to transform the management of young patients with breast cancer at high risk of relapse and to improve their quality of life after the disease. This innovative, large-scale programme is based on a major international multi-centre clinical trial and benefits from the collaboration of 26 partners, including cancer centres, universities, international cooperative research groups and patient associations based in 15 different countries. It is funded by the European Union to the tune of €9.5 million (grant agreement no. 101156800).
When it occurs before menopause in women under the age of 50, hormone-dependent and HER2-negative breast cancer represents a major challenge, not least because of the impact of treatment on life after the disease. Although current therapies are effective, they can be highly toxic, particularly chemotherapy, which can have long-lasting side effects and significantly alter patients' quality of life. 25% of women diagnosed with this cancer are under 50 in the European Union (and up to 55% in some countries).
While tools exist to determine the level of risk and avoid chemotherapy (women at low and intermediate risk), some high-risk patients could also safely forego chemotherapy, while receiving optimal endocrine treatment combining oral hormone therapy and suppression of ovarian function.
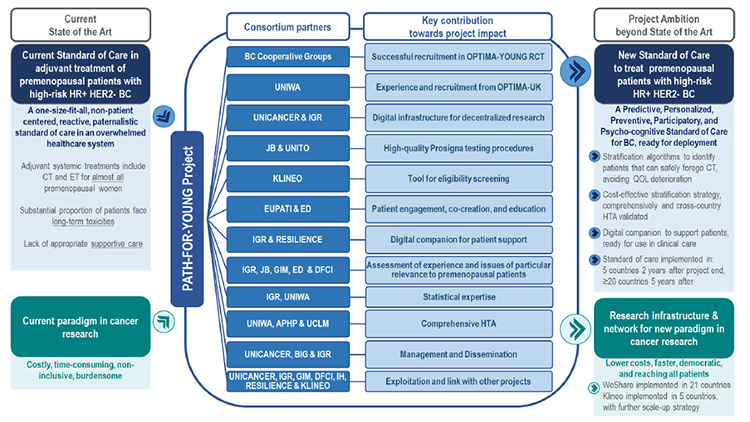
The Path-For-Young project thus aims to establish and implement a new standard of personalised care based on:
- Reducing chemotherapy by identifying, through a multi-parameter test, women for whom chemotherapy could be safely avoided while ensuring treatment efficacy and healing opportunities. To this end, the project will build on the OPTIMA-UK randomised controlled clinical trial (4,500 patients included, including 1,620 pre-menopausal patients) and expand it with the implementation of a global pragmatic randomised clinical trial: OPTIMA-YOUNG (3,380 women) sponsored by Unicancer.
- Improving quality of life: Combined with the reduction in chemotherapy and the integration of digital tools and individualised monitoring, the project places patients at the heart of the process, offering ongoing support and solutions tailored to their needs.
With more than 300 centres across Europe, America, and Oceania, as well as 50 in France, Path-For-Young relies on a multidisciplinary team of experts from various backgrounds. Recruitment of nearly 3,400 patients will begin in 2025, with follow-up planned over several years.
In summary, the objective of Path-For-Young is to:
- Predict the benefit of chemotherapy for pre-menopausal patients.
- Customise adjuvant treatment choices.
- Prevent deterioration in quality of life by avoiding the long-term effects of treatment.
- Improve supportive care strategies, including through better use of digital health tools.
The project brings together a multidisciplinary consortium that includes the expertise of:
- Clinicians (oncologists, pathologists, supportive care specialists).
- Epidemiologists.
- Patients, caregivers and patient associations.
- Experts in human and social sciences (HSS) (sociologists, psychologists, ethics, communication).
- Digital technology specialists.
- Health economists - health technology assessment.
A project supported by the European Union
This project is supported by European Union grant no. 101156800.
Path-For-Young marks an essential milestone in breast cancer management and paves the way for more personalised, quality-of-life-based medicine after illness.
List of partners:
- Gustave Roussy - France
- UNICANCER (Coordinator) - France
- Breast International Group, B.I.G. AISBL - Belgium
- Universita Degli Studi di Torino - Italy
- Institut Jules Bordet ASBL - Belgium
- Stichting EUPATI Foundation – The Netherlands
- The University Of Warwick - United Kingdom
- Résilience - France
- Elliniki Synergazomeni Ogkologiki Omada - Greece
- Cancer Trials Ireland Company Limited By Guarantee - Ireland
- Grupo Argentino de Investigacion Clinica en Oncologia - Argentina
- Grupo de Estudios Clínicos Oncologico Peruano - Peru
- Gruppo Oncologico Italiano Di Ricerca Clinica – Italy
- Europa Donna The European Breast Cancer Coalition - Italy
- Europa Donna Slovensko Zdruzenje Za Boj Proti Raku Dojk - Slovenia
- Assistance Publique Hopitaux de Paris APHP - France
- Innovandum Health SL - Spain
- Grupo Español de Estudio, Tratamiento y Otras Estrategias Experimentales en Tumores Sólidos – Spain
- Central and East European Oncology Group - Poland
- Dana-Farber Cancer Institute - USA
- Universidad De Castilla - La Mancha - Spain
- Clinical Research Technology SRL - Italy
- KLINEO - France
- Instituto D'Or de Pesquisa e Ensino – Brazil
- Latin American Cooperative Oncology Group - Brazil
- ANZ Breast Cancer Trials - Australia/New Zealand